Work Done By A System
Work done by a system. Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments. Thermodynamics is a branch of physics which deals with the energy and work of a system. Work Done by a System.
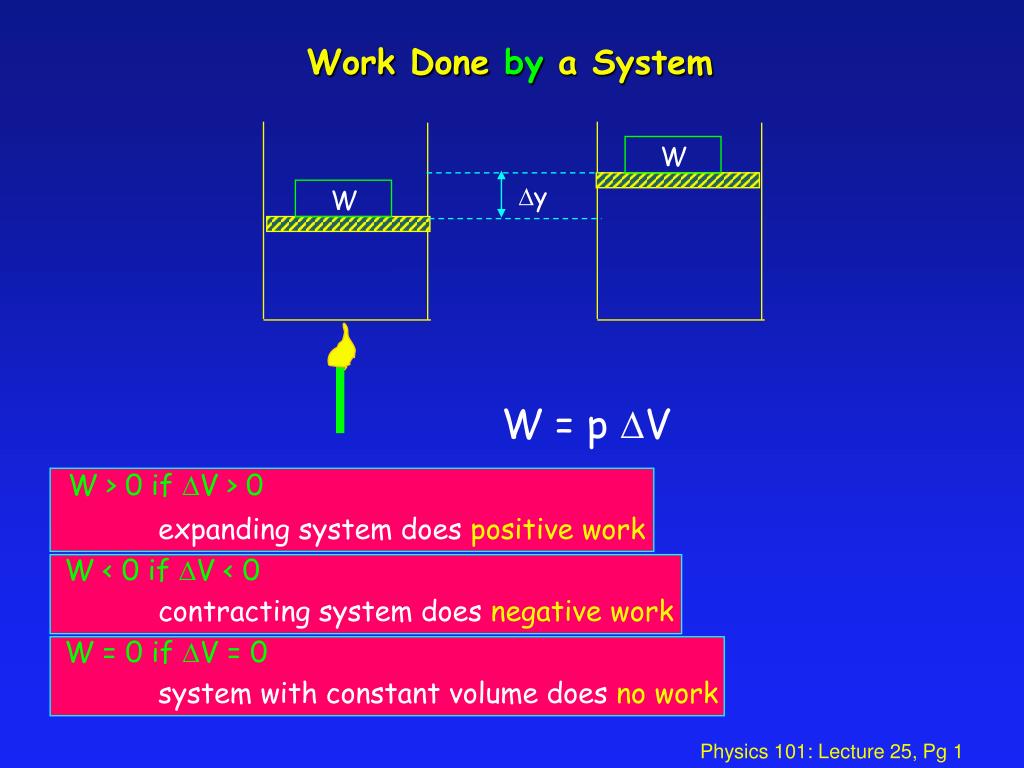
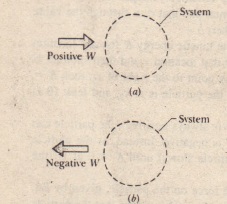
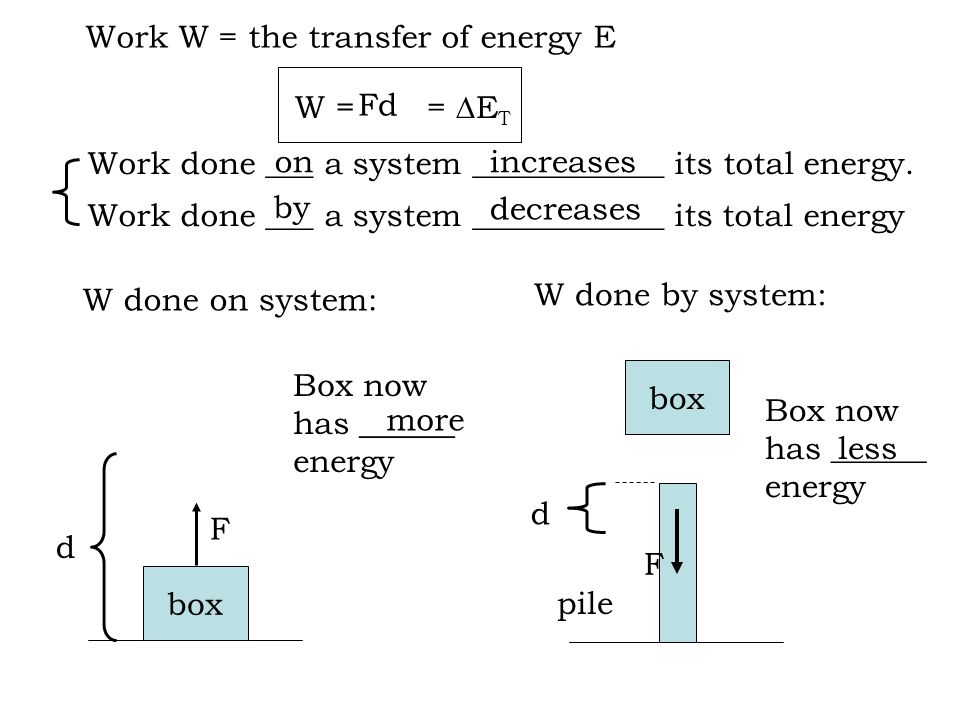
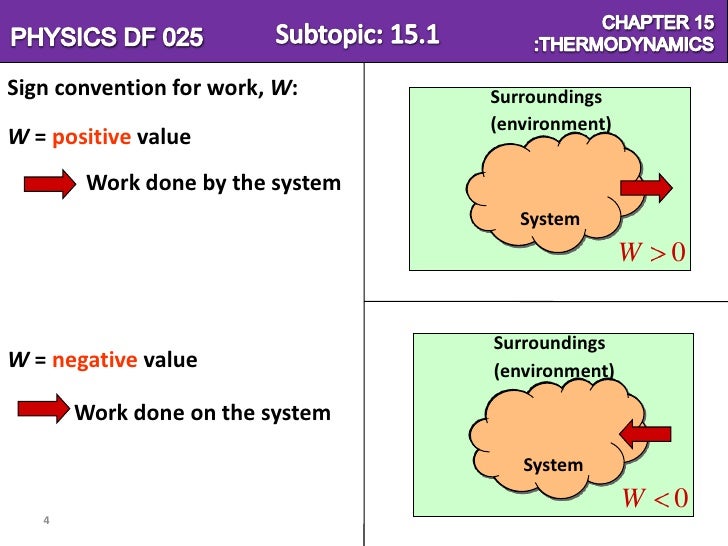
More specifically work is the energy transfer associated with force acting through a distance. An electric motor chemical work surface tension work elastic work. Work done by the system is positive.
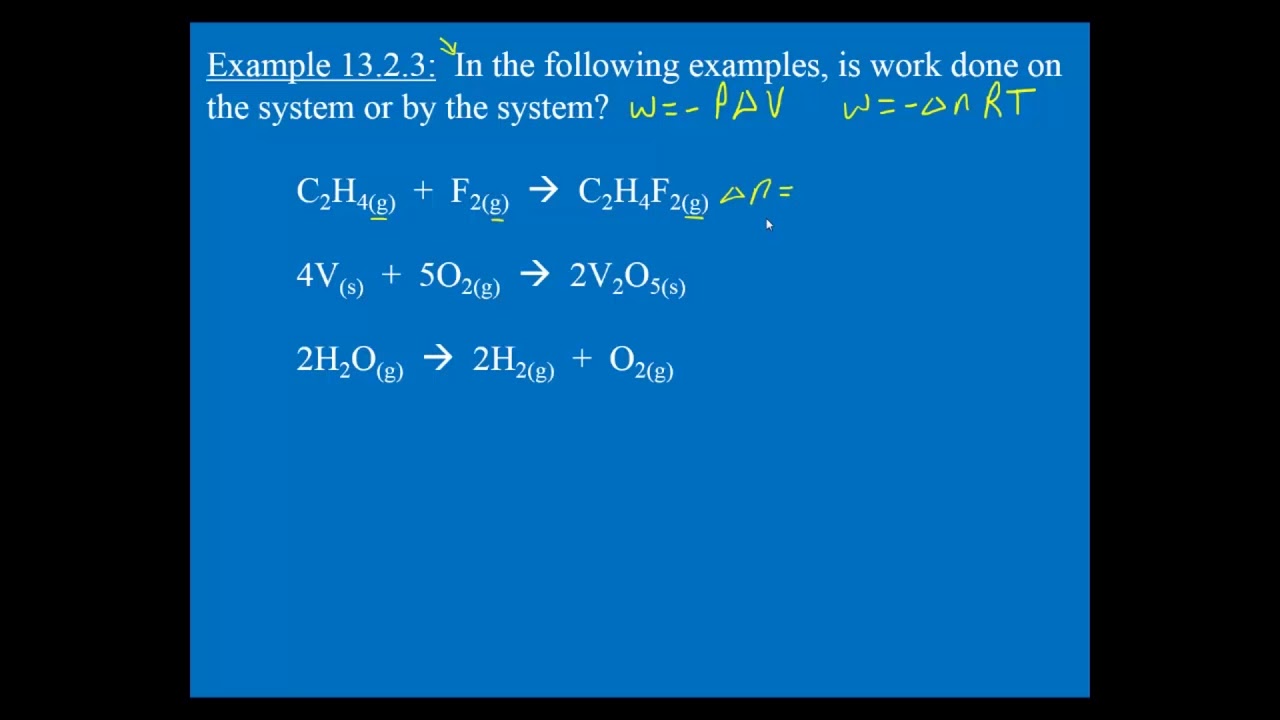
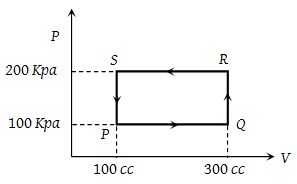
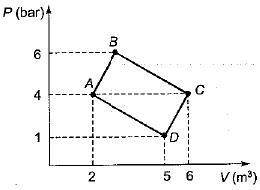
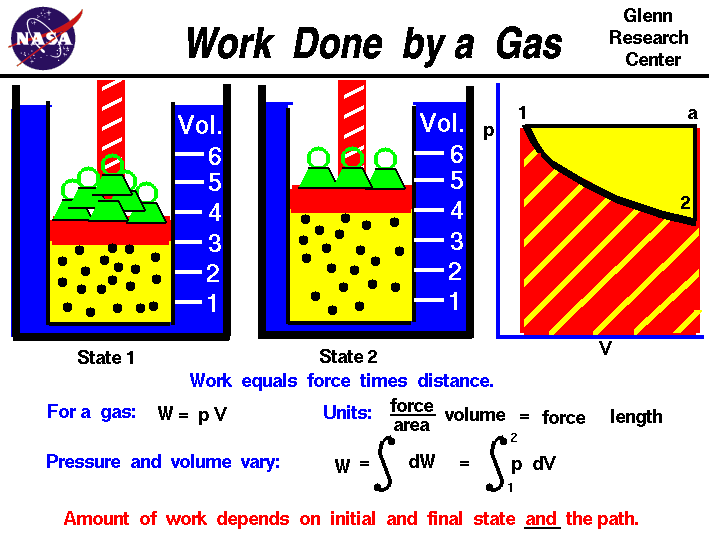
Although work done on a system can be any type of work there are a couple of things you should keep in mind with this question. 3 Work VW S B. The element of work W done by a system is given by W p V where p is the pressure from PHY 455 at Southern New Hampshire University.
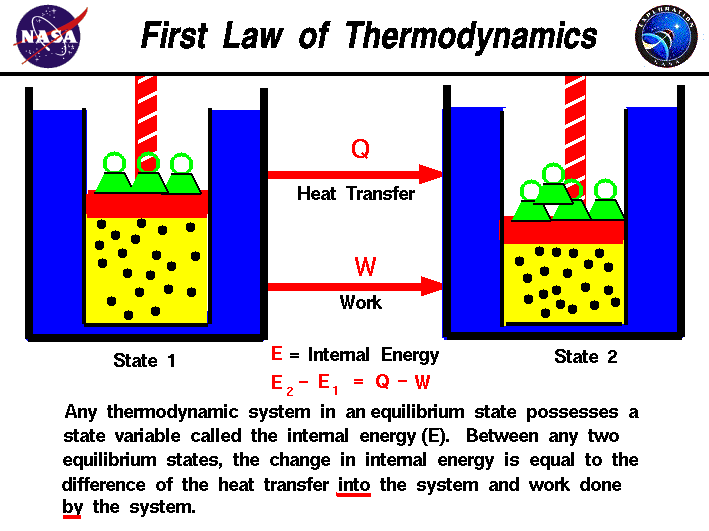
Then how does a thermodynamic system do work. 2 d U d Q d W. Here the first law is written as.
Work is a form of energy but it is energy in transit. Rest of the arguments follow as above. There are two types of things in the science world - the system and the surroundings.
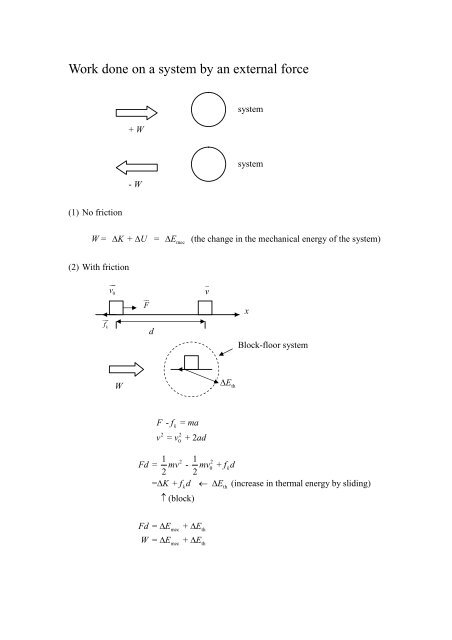
So W 1 0. A system contains no work work is a process done by or on a system. Any other means for changing the energy of a system is called workWe can have push-pull work eg.
Each conservative force can be identified with a potential energy and. In thermodynamics work performed by a system is the energy transferred by the system to its surroundings.
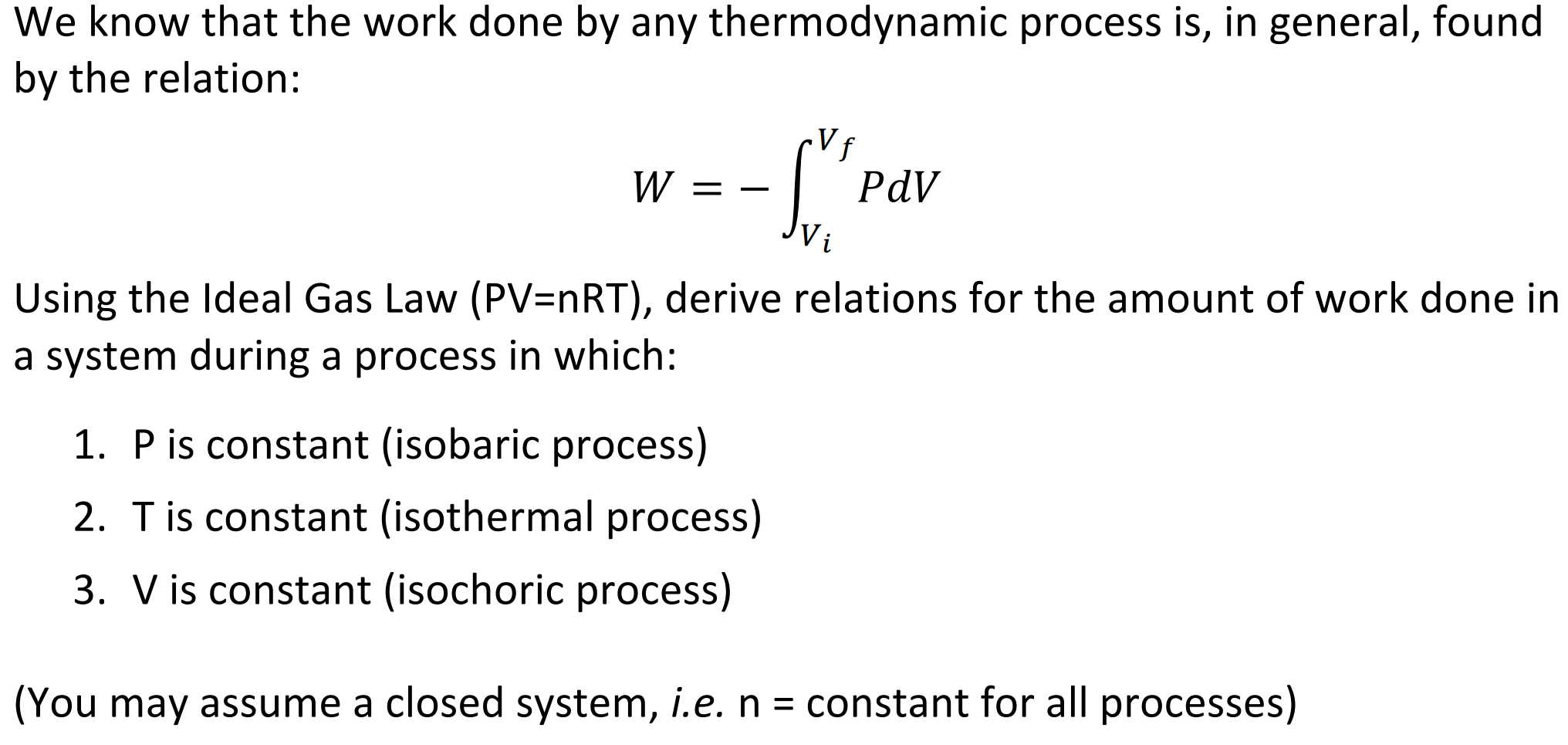
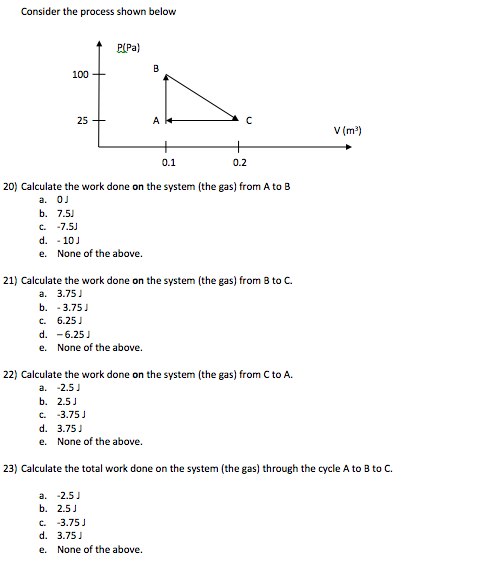
Thus the work done W 2 for horizontal path will be W 2 -pdV negative sign is due to work is done on the gas - 15 Pa 4m 3 -60 Pam 3 -60 Pam 3 1 J1 Pam 3 -60 J.
The work done by a gas at constant pressure is. Kinetic energy potential energy and internal energy are forms of energy that are properties of a system. 41-46 Section 131 stated that heat is a way of changing the energy of a system by virtue of a temperature difference only. What about the. An example of this system is a gas in a box with fixed walls. Work is a form of energy but it is energy in transit. Work done by system therefore. Thus the work done W 2 for horizontal path will be W 2 -pdV negative sign is due to work is done on the gas - 15 Pa 4m 3 -60 Pam 3 -60 Pam 3 1 J1 Pam 3 -60 J. If work is applied to the system d W term becomes negative making two negatives positive which is identical to equation 1 and heat added to the system is still positive here.
The work done is zero. When the system does work internal energy U is negative because the system has lost energy by working against the surrounding. Then how does a thermodynamic system do work. In thermodynamics work is defined as -p_exDelta V for an ideal gas. Kinetic energy potential energy and internal energy are forms of energy that are properties of a system. 3 Work VW S B. Work is done BY the system during the power stroke ie.



















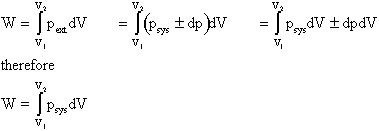





Post a Comment for "Work Done By A System"